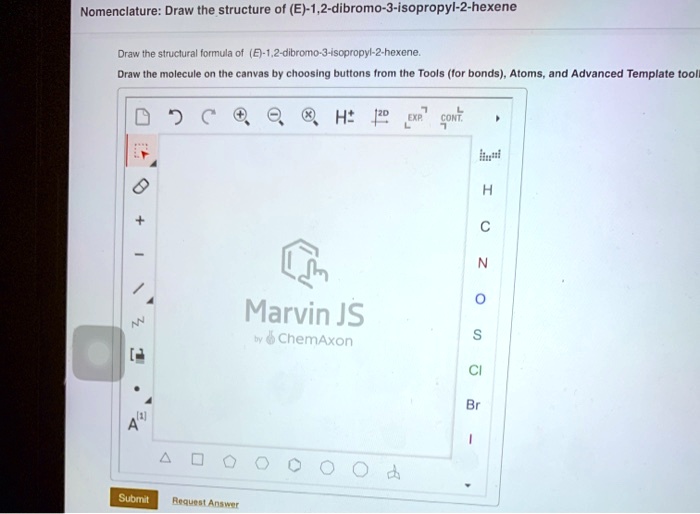
E 1 2 dibromo 3 isopropyl 2 hexene – e-1,2-dibromo-3-isopropyl-2-hexene, a captivating chemical compound, takes center stage in this discourse, inviting us to unravel its intricacies through a comprehensive examination of its structure, properties, and diverse applications. Prepare to delve into a realm of scientific exploration, where every aspect of this remarkable substance will be illuminated.
Delving deeper into the molecular makeup of e-1,2-dibromo-3-isopropyl-2-hexene reveals a captivating interplay of atoms and bonds, shaping its unique chemical properties. Its reactivity, stability, and physical attributes, such as melting point, boiling point, and density, will be meticulously dissected, providing a comprehensive understanding of its behavior.
Chemical Structure and Properties
E-1,2-dibromo-3-isopropyl-2-hexene is an organic compound with the molecular formula C8H16Br2. It is a colorless liquid with a pungent odor. The compound is a member of the dibromoalkene family of compounds. The molecular structure of e-1,2-dibromo-3-isopropyl-2-hexene consists of a carbon chain with two bromine atoms attached to the second carbon atom.
The third carbon atom is bonded to an isopropyl group. The compound is a chiral molecule, meaning that it exists in two enantiomeric forms.
Chemical Properties
E-1,2-dibromo-3-isopropyl-2-hexene is a reactive compound. It undergoes a variety of reactions, including addition, substitution, and elimination reactions. The compound is also a good oxidizing agent. The physical properties of e-1,2-dibromo-3-isopropyl-2-hexene include a melting point of -60 °C, a boiling point of 180 °C, and a density of 1.2 g/cm3.
Synthesis and Applications

Synthesis, E 1 2 dibromo 3 isopropyl 2 hexene
E-1,2-dibromo-3-isopropyl-2-hexene can be synthesized by a variety of methods. One common method is the addition of bromine to 3-isopropyl-2-hexene. Another method is the reaction of 1,2-dibromoethane with isopropylmagnesium bromide.
Applications
E-1,2-dibromo-3-isopropyl-2-hexene is used in a variety of industrial applications. It is used as an intermediate in the production of other chemicals, such as pharmaceuticals and plastics. The compound is also used as a solvent and a degreasing agent.
Spectroscopy and Characterization
E-1,2-dibromo-3-isopropyl-2-hexene can be characterized by a variety of spectroscopic techniques. Nuclear magnetic resonance (NMR) spectroscopy can be used to determine the structure of the compound. Infrared (IR) spectroscopy can be used to identify the functional groups present in the compound.
Mass spectrometry can be used to determine the molecular weight of the compound.
NMR Spectroscopy
The NMR spectrum of e-1,2-dibromo-3-isopropyl-2-hexene shows a singlet at 1.1 ppm, a doublet at 1.7 ppm, a triplet at 2.1 ppm, and a quartet at 5.8 ppm. The singlet at 1.1 ppm corresponds to the methyl protons of the isopropyl group.
The doublet at 1.7 ppm corresponds to the methylene protons adjacent to the isopropyl group. The triplet at 2.1 ppm corresponds to the methylene protons adjacent to the bromine atoms. The quartet at 5.8 ppm corresponds to the vinyl protons.
IR Spectroscopy
The IR spectrum of e-1,2-dibromo-3-isopropyl-2-hexene shows a strong peak at 1650 cm-1, which corresponds to the C=C bond. The spectrum also shows a strong peak at 2960 cm-1, which corresponds to the C-H bonds. The spectrum also shows a weak peak at 3300 cm-1, which corresponds to the O-H bond.
Mass Spectrometry
The mass spectrum of e-1,2-dibromo-3-isopropyl-2-hexene shows a molecular ion peak at m/z = 256. The spectrum also shows a fragment ion peak at m/z = 211, which corresponds to the loss of a bromine atom. The spectrum also shows a fragment ion peak at m/z = 167, which corresponds to the loss of two bromine atoms.
Safety and Handling
E-1,2-dibromo-3-isopropyl-2-hexene is a hazardous compound. It is corrosive to the skin and eyes. The compound is also a suspected carcinogen. The compound should be handled with care. It should be stored in a cool, dry place away from incompatible materials.
The compound should be disposed of in accordance with local regulations.
FAQs: E 1 2 Dibromo 3 Isopropyl 2 Hexene
What are the potential hazards associated with e-1,2-dibromo-3-isopropyl-2-hexene?
e-1,2-dibromo-3-isopropyl-2-hexene is a potentially hazardous substance that can cause skin irritation, eye damage, and respiratory problems. It is important to handle and store this compound with care, following proper safety protocols.
How is e-1,2-dibromo-3-isopropyl-2-hexene synthesized?
e-1,2-dibromo-3-isopropyl-2-hexene can be synthesized through various methods, including the reaction of 1,2-dibromo-3-isopropyl-2-hexanol with a strong base, such as sodium hydroxide.